If you've been riding the health wave, you've probably come across the term oxidative stress a few times. You may also have heard that antioxidants protect the body against oxidative stress. But what exactly is oxidative stress and what function do antioxidants have in this connection?
What is oxidative stress - chemically speaking?
As the name suggests, oxidative stress means that something is oxidized, but to understand what oxidation really is, we start with oxidation and reduction processes, which are called Redox reactions for short.
A redox reaction is a reaction in which one chemical compound is oxidized at the same time as another chemical compound is reduced. That is that an atom in a chemical compound receives electrons (reduced, because the substance goes down one oxidation step) from another atom in another chemical compound, which thus gives up electrons (oxidises).
Just such reactions are the very chemical process behind oxidative stress. This means that an electron transfer takes place between atoms or molecules.
Redox reactions take place all the time in our body and are an important prerequisite for the body to function. For example, photosynthesis, which we know from plants, and respiration are also redox reactions. The same applies to our metabolism, where starch acts as a reducing agent and oxygen as an oxidizing agent.
Why is oxidative stress a problem?
Oxidation is a necessity for the body, but the problem arises when the oxidation process starts a chain reaction. It happens when a strong oxidizer, such as oxygen (dioxygen), oxidizes fatty acids, DNA, proteins or metals. The oxidation creates free radicals – i.e. oxidized substances which now lack electrons and therefore become extremely reactive. These free radicals now begin to oxidize other atoms and molecules, creating more free radicals, thus starting a chain reaction.
"Oxidative stress a chain reaction of electron transfers between atoms or molecules"
This chain reaction can damage our DNA, cells and cell membranes, which can be the cause of a number of diseases, eg cardiovascular diseases, cancer and other degenerative diseases. Free radicals also affect our aging process.
Oxidation is a natural part of the body's processes and is in many cases a necessity for the body to function. The problem arises when there is an imbalance in the body between the free radicals and the amount of antioxidants.
Antioxidants – stop the chain reaction
Antioxidants are used to reduce or stop the oxidation processes due to oxidative stress. Antioxidants are chemical compounds that can give up electrons or bind to free radicals without becoming reactive themselves. This stops the chain reaction.
A good example that illustrates the effect of antioxidants is to take an apple and cut it in half. One half is smeared with lemon juice and the other is left alone. After a short time, the oxidation processes begin in the apple without lemon juice, and the surface turns brown. But because lemon juice contains antioxidants, it takes longer for the second piece of apple to start turning brown. This is because the oxidation processes first use up all the antioxidants before the oxidation affects the apple itself.

During stress and hard physical training, the body uses extra amounts of antioxidants to stop the oxidative stress. That is why it is important to get a lot of antioxidants.
How do I get antioxidants?
Many vitamins, minerals and amino acids are antioxidants or have an antioxidant effect in interaction with other vitamins, minerals and nutrients. The most important antioxidants are i.a. Vitamin A , vitamin C , vitamin B6 , vitamin E , selenium and zinc . Many amino acids also have an antioxidant effect and here, for example, Glutamic acid, Lutein, Lycopene, Quercetin and Leucoanthocyanin are mentioned.
"The amount of antioxidants in the body is directly proportional to our lifespan"
MD Richard Cutler
Many of the antioxidants are found naturally in e.g. fruit and vegetables as well as in nuts and whole grains, and is thus very easily available in a healthy and varied diet. However, most vitamins are very fragile in the face of the processing and preparation that we subject much of our food to, and it may therefore be a good idea to supplement the diet with nutritional supplements.
Another thing that is important to remember is that vitamins, minerals and other nutrients are interdependent and work as a complex. It is therefore not enough to eat a single antioxidant, but you should instead consume several different ones.
So what should I do to reduce oxidative stress?
In addition to the reactive compounds that are formed during normal metabolism in the body, sunlight and ionized radiation can trigger processes that lead to the formation of free radicals. In addition, it contains the following free radicals:
- Smoke
- Exhaust from cars
- Polluted air
- Certain medicines
- Pesticides
- Solvents
- UV rays from the sun
In addition, general stress, poor diet and poor sleep can also affect the production of free radicals and have a negative impact on oxidative stress.
It is therefore a very good idea to live on a healthy and varied diet with plenty of fruit and vegetables, as well as whole grains and nuts. In addition, one should consider taking various vitamin and mineral supplements to supplement the diet with antioxidants. This is especially a good idea if you do hard training, often have a stressful everyday life, smoke or live in areas with high air pollution.
WORD LIST:
-
Redox reaction: a reaction in which an electron is transferred from one atom/molecule to another atom/molecule. An example of a Redox reaction could be Zinc (Zn) and Nitrate (NO 3 - ), which in an alkaline solution become Zinc, which lacks 2 electrons (Zn 2+ ) and Nitrite (NO 2 ).
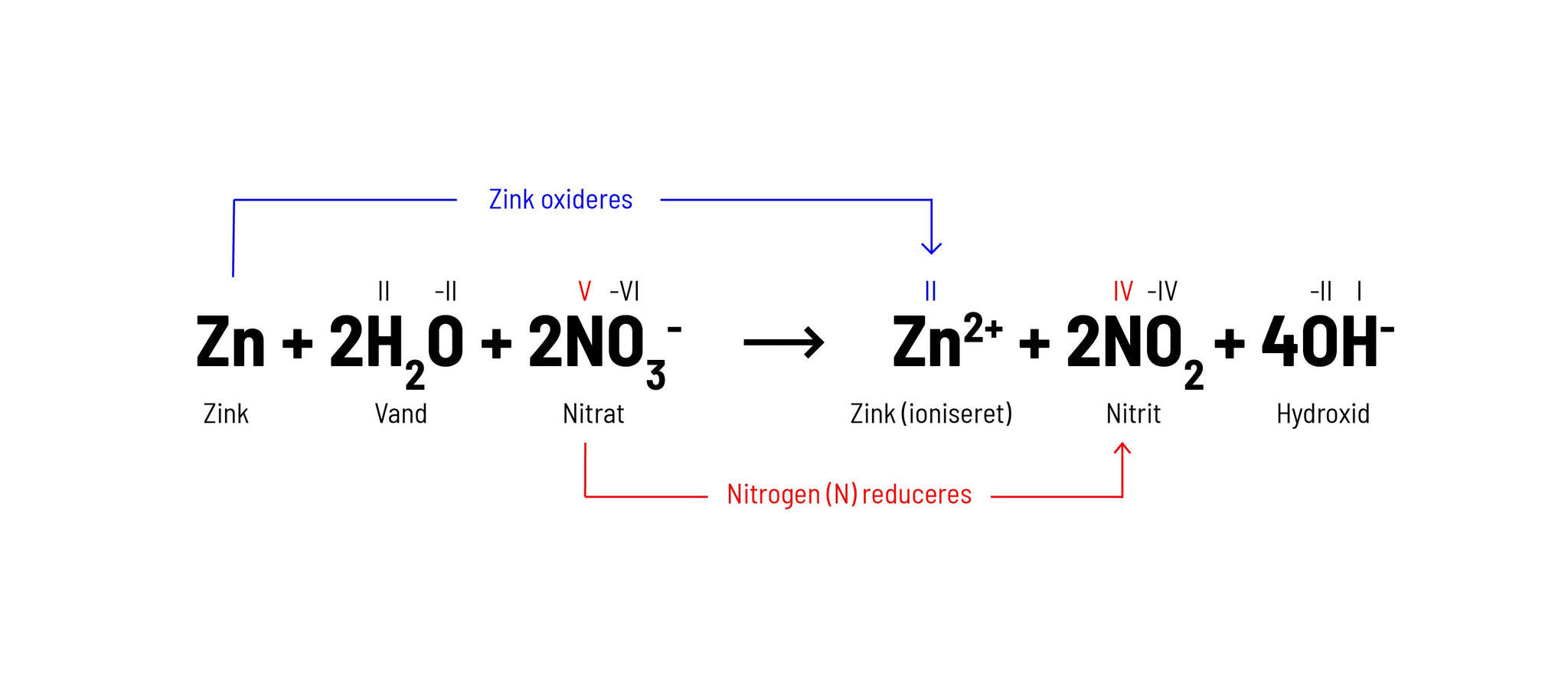 In this reaction, Zn is a reductant because zinc donates 2 electrons to the 2 N (nitrogen) atoms, and thus the oxidation state of the 2 N atoms is reduced from +5 to +4 upon receiving the electrons. This makes N an oxidizer as this electron transfer oxidizes Zn.
In this reaction, Zn is a reductant because zinc donates 2 electrons to the 2 N (nitrogen) atoms, and thus the oxidation state of the 2 N atoms is reduced from +5 to +4 upon receiving the electrons. This makes N an oxidizer as this electron transfer oxidizes Zn.
-
Oxidizer: Atom/molecule that has caused another atom/molecule to give up electrons (oxidize)
-
Reducer: Atom/molecule which has caused another atom/molecule to receive electrons (reduced)
-
Oxidation state: a way of keeping track of an element's electrons. It corresponds to the charge an element would have if all its bonds and/or ligands were removed. The oxidation state is written in Roman numerals.
-
Free radicals: A radical or a free radical is a term for an atom or compound having unpaired electrons or an incompletely filled electron shell. This makes them extremely reactive.
Sources:
"Antioxidants and health", Exercise and Nutrition Council, 2006
"Live healthy with vitamins and minerals", Henrik Dilling, Lindhardt og Ringhof Forlag A/S, 2016, 3rd edition, 1st edition, pp. 182-185
https://www.youtube.com/watch?v=v5sDNmYCaqo
https://da.wikipedia.org/wiki/Radikal_(chemistry)
http://denstoredanske.dk/It,_teknik_og_naturvidenskab/Kemi/Elektrokemi/oxidatio
http://denstoredanske.dk/It%2c_teknik_og_naturvidenskab/Kemi/Elektrokemi/redoxproces

 In this reaction, Zn is a reductant because zinc donates 2 electrons to the 2 N (nitrogen) atoms, and thus the oxidation state of the 2 N atoms is reduced from +5 to +4 upon receiving the electrons. This makes N an oxidizer as this electron transfer oxidizes Zn.
In this reaction, Zn is a reductant because zinc donates 2 electrons to the 2 N (nitrogen) atoms, and thus the oxidation state of the 2 N atoms is reduced from +5 to +4 upon receiving the electrons. This makes N an oxidizer as this electron transfer oxidizes Zn.